A Peer to Peer Research Atmosphere Designed to Encourage and Stimulate Product Research While Maximizing Cost Efficiency
CYGNOS Biotech is conducting multiple clinical studies, including pilot and pivotal trials, in order to support safety and bio-efficacy for several viral disease indications for dogs, cats, and horses to meet FDA New Animal Drug guidance requirements. The Company is also seeking investigators to participate in clinical studies to treat canine malignant melanoma.
The INAD number opens a file with the FDA CVM allowing us to perform preliminary efficacy and safety studies of the drug in companion animals. Such pilot studies allow us to develop the final conditions or protocol for the pivotal study for review and final approval before initiation of the pivotal clinical study.
- Canine Upper Respiratory Disease Complex (including distemper viral infections) click here to learn more about the Distemper Pilot Clinical Study
- Feline Upper Respiratory (URI) Disease by Feline Herpes Virus (FHV-1)
- Feline Corona Virus (FCcoV) infections
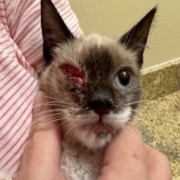
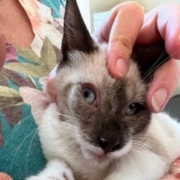
- Feline Ophthalmic Infections by FHV-1, FCoV click here to learn more about the Ophthalmic Pilot Clinical Study
- Stage II and Stage III Oral Canine Malignant Melanoma
A formal collaboration with CAAT Friends Rescue Network provides eligible feline subjects for feline URI and ophthalmic infections for pilot studies. Similar agreements have been made with veterinarians to provide pilot studies.
If you have an interest in seeking further information or in participating in these clinical studies or trials, please contact us.
Canine Distemper Disease Complex
Feline Herpes Virus Ophthalmic Infection