Successful Canine Distemper Disease Pilot Clinical Study
Pilot Clinical Trial Sites
- Denton, TX – 1 site (45 dogs)
- Los Angeles, CA – 3 sites (31 dogs)
- Austin, TX – 1 site (1 dog)
Treatment
- Daily subcutaneous injections at a dosage of 40-60μg/kg given for a minimum of 28 days.
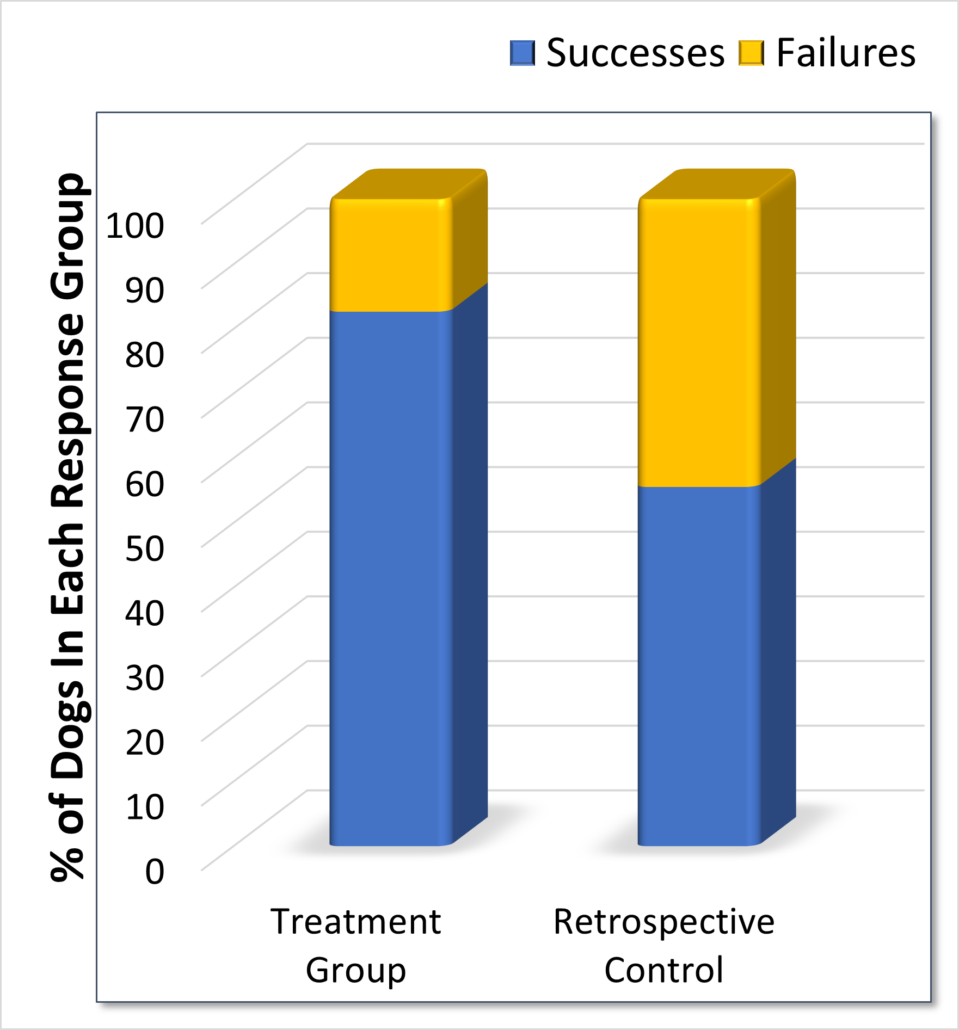
Results
Treatment Group
-
- A survival rate of 83% or 62 out of 75 animals with a P value of <0.05 as compared to the retrospective control.
- Two additional animals died from non-related distemper causes
Retrospective Control
-
- The literature indicates that canines treated with standard of care (SOC) – 50% or less do not survive.
- All treated puppies aged twelve (12) months or less survived – Literature indicates that puppies 12 months or less 100% usually don’t survive.
Kelly, dumped by her owners, overcomes distemper, lands in loving home: People and their pets
BEREA, Ohio — Such a tiny thing she is. Such a huge heart she has – one that she shares with everybody. CYGNOS Biotech partnered with a local Ohio organization to offer life-saving treatment to an abandoned dog and her puppies suffering from Distemper.
When we met her, Kelly greeted Pam and me as if we were long, lost friends. We didn’t know it then, but that’s how she treats everyone: humans, dogs – cats, even. (Squirrels, rabbits, and whatnot are another story. She’s as sweet as pie. But she is, after all, a dog).
If you have an interest in seeking further information or in participating in these clinical studies or trials, please contact us.


